Summary of 2010 MMIG Meeting
March 5, 2010
American Academy of Dermatology Annual Meeting
Miami, FL
Prepared by Drs. Jayasri Iyer & Paul Nghiem
Speakers/Topics:
- Brief updates on MCC topics
i) Consensus staging system ii) ICD9 codes iii) Prospective trials in MCC.- Dr. Paul Nghiem, U Washington, Seattle
- Update on the Merkel cell polyomavirus
- Dr. Patrick Moore, U of Pittsburgh
- Update on activity and multi-institutional trials of anti-CD56 mAb therapy for MCC
- Jim O’Leary, Bob Lutz, Kathy Whiteman, Immunogen, Waltham MA
- Insights into the use of brachytherapy* & PET-CTs in managing MCC
(*surface-mold computer-optimized high-dose-rate brachytherapy)- Dr. Linda Wang, DFCI, Boston
- Development of an IL-12 intra-tumoral electroporation trial in MCC
- Siegrid Yu, U of California, San Francisco
Goals of MMIG
- Promote communication and collaborative studies on MCC
- Enhance access to patient data and specimens
- Expand evidence-based care for MCC
MMIG is funded in part by a grant from the Jerry Wachter Fund of the American Cancer Society (www.jw.org).
Please note that in some cases these summaries reflect unpublished data and are provided to help MMIG members manage their patients and give an overview of what is being done at different centers for care and research.
1) Brief updates on MCC topics in 2010:
Dr. Paul Nghiem, U Washington, Seattle
i) Consensus staging system:
A new consensus staging system for MCC has been adopted for use in 2010. This staging system has been derived from 5,823 MCC patients from the National Cancer Database and is outlined as follows:
Stage I: Local, ≤ 2cm
Ia: Nodes microscopically negative and not clinically detectable
Ib: Nodes not clinically detectable (no pathologic eval of nodes done)
Stage II: Local, > 2cm
IIa: Nodes microscopically negative and not clinically detectable
IIb: Nodes not clinically detectable (no pathologic eval of nodes done)
IIc: Primary tumor invading bone/muscle/fascia/cartilage
Stage III: Regional Nodal Disease
IIIa: Micrometastasis
IIIb: Macrometastasis (clinically detectable)
Stage IV: Distant Metastatic Disease
The major changes from existing systems are inclusion of sub-stages for method of nodal evaluation (clinical evaluation vs. pathologic evaluation) and for clinically apparent vs only microscopic nodal involvement. Also, 2.0 cm tumors are now included in Stage I rather than Stage II.
References:
Lemos B, Storer B, Iyer J, Phillips JL, Bichakjian CK, et al. “Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: Analysis of 5,823 cases as the basis of the first consensus staging system for this cancer.” J Am Acad Dermatol. In Press.
“Merkel cell carcinoma”, Chapter 30, AJCC Cancer Staging Manual, 7th Edn, Springer, 2009
ii) ICD9 codes:
Eight new ICD9 codes for Merkel cell carcinoma were introduced in October 2009 and are shown below. These codes will improve our ability to obtain insurance approval for procedures for MCC patients, as well as track costs, incidence, etc, for this cancer.
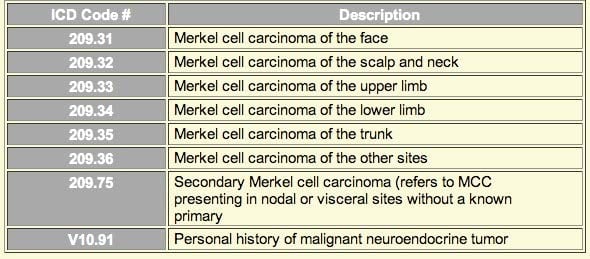
iii) Prospective trials in MCC:
Several international conference calls were held over the past year to discuss whether randomized trials could be designed to address optimal management of MCC with the existing modalities of surgery, radiation and chemotherapy. Many of you participated in them. Unfortunately, there are several issues that likely mean that multi-national, multi-institutional, prospective randomized clinical trials are not likely feasible for MCC:
- Great variability in treatment practices among clinicians and hesitation to randomize patients for trials
- Logistical and financial issues would be challenging (although not prohibitive)
- Power calculations indicated that many hundreds of patients would need to be randomized to answer questions such as in which patients adjuvant radiation is or is not needed.
Instead of using a randomized approach, we may need to work to optimize standard therapy by analyzing outcomes from various prospective cohorts in whom multiple treatment/risk parameters are closely captured, ideally in multi-institutional/multi-national collaborations. This will likely be the focus as we move forward in the future. Once again, individual centers are encouraged to capture their patients prospectively and track their treatment, clinical parameters and outcomes. We are happy to provide example data intake forms and IRB applications that could be adapted for use at your institution to track MCC patients.
2) Update on the Merkel cell polyomavirus
Dr. Patrick Moore, U of Pittsburgh
Dr. Moore briefly described his team’s discovery of the Merkel cell polyomavirus (MCPyV) by the digital transcriptome subtraction method. He summarized updates on this novel virus.
- MCPyV is present in ~ 80% MCC tumors
- MCPyV is specific for MCC
- T antigen (likely an oncoprotein) is persistently expressed in most MCC tumors
- Monoclonal antibody staining against the MCPyV-specific region of the large T antigen (CM2B4) stains only MCC tumors (not SCC or BCC or other malignancies).
- Prevalence of MCPyV is common and 50-80% of healthy controls have antibodies to MCPyV VP1 capsid protein as compared to ~90% MCC patients.
Even though MCPyV is a very common virus that likely infects humans in early childhood, MCC itself is rare. Dr. Moore explained that two rare mutations are required to occur in the same cell: i) integration of the virus in the tumor genome, ii) deletion of detrimental portions of the integrated T antigen that would prevent survival of the cell due to genomic instability.
Dr. Moore’s group has found that persistent expression of T antigen is required for growth and survival of MCC cell lines that are positive for the virus, providing additional evidence that this virus is causal for 70-80% of MCC.
3) Update on activity and multi-institutional trials of anti-CD56 mAb therapy for MCC
Jim O’Leary, Bob Lutz, Kathy Whiteman, Immunogen, Waltham MA
Dr. O’Leary discussed the results of phase 1 trials of IMGN901 (anti-CD56 mAb linked to a maytansinoid toxin) for neuroendocrine cancer. All Merkel cell carcinoma patients had CD56 positive tumors (95% of MCC tumors are CD56 positive).
- Criteria for enrolling patient in this trial: MCC patients with recurrent/progressive disease who have recurred after at least one prior chemotherapy regimen, MCC tumor should be CD56 positive
- Treatment: 1 cycle of IMGN = 3 weeks treatment; maximum number of cycles treated: 6
- Primary end point: response at the end of each cycle or every other cycle as measured by CT scan or MRI
- Results among six patients:
- 1 patient had partial remission at the end of cycle 1 and complete remission at cycle 3. Patient tolerated all 6 cycles well and is in clinical remission for more than 4 years.
- 1 patient had partial remission at the end of cycle 1. This was confirmed 9 weeks later with a repeat MRI. However the patient developed “Reversible Posterior Leukoencephalopathy Syndrome” (RPLS). A cluster of syndromes such as headaches, confusion, seizures and visual disturbances characterizes RPLS. It is associated with transient, predominantly posterior cerebral lesions revealed by neuroimaging. IMGN901 was not continued however patient continued to derive benefit 10 weeks after the 1st cycle of treatment and at one year is considered a clinical complete response.
- c) 1 patient had stable disease for 3 months following treatment with IMGN901
- Responses to IMGN901 were seen within the first cycle itself (i.e. 3 weeks)
Currently, the following sites are open for enrollment in this clinical trial:
- Christie Hospital NHS Trust (UK) – Dr. Paul Lorigan
- Royal Marsden Hospital (UK) – Dr. Mary O’Brien
- Weston Park Hospital (UK) – Professor Penella Woll
- The University of Texas MD Anderson Cancer Center – Dr. Frank Fossella
- The Ohio State University – Dr. Miguel Villalona
Sites opening in 2010:
- Fred Hutchinson Cancer Research Center, Seattle
- Nevada Cancer Institute
- Dana Farber Cancer Institute
4) Insights into the use of brachytherapy* & PET-CTs in managing MCC
*surface mold computer optimized high dose rate brachytherapy
Dr. Linda Wang, DFCI, Boston
i) Brachytherapy for extensive regional cutaneous MCC
Dr Wang discussed the use of surface-mold computer-optimized high-dose-rate (HDR) brachytherapy (SMBT) for in-transit and retrograde cutaneous metastases on the lower extremities, which is unfortunately common despite aggressive management. The diffuse nature of theses metastases often renders negative margin excision impossible. Response to chemotherapy is limited and rarely durable. External beam radiation therapy (EBRT) is technically challenging given the size of the radiation target and the complex curvatures of the lower extremities, which can result in marked dose heterogeneity within the target volume.
Brachytherapy refers to short-range radiation therapy where radioactive sources are placed within or very close to the target of interest. A moldable applicator composed of a thermoplastic cast and hollow HDR treatment catheters is created. A single 1 x 3 mm radioactive seed of the gamma-emitter Iridium-192 is directed to so called “dwell” positions within the hollow treatment catheters. The location of the dwell positions and the amount of time the radioactive source spends at each position is computer-optimized to provide the most uniform radiation dose to the target area.
Benefits:
- Radiation dose delivered is matched to the shape & curvature of the surface being treated (e.g., “conformal” radiotherapy)
- A homogeneous dose distribution is achieved through the target volume
- A sharp dose fall-off at the edge of the treatment field minimizes unnecessary exposure to adjacent skin and underlying normal structures
- Superb tissue tolerance with minimal treatment related morbidity and no increase in lymphedema
- Durable response
Reference: Cotter SE, Devlin PM, Sahni D, Hansen JL, O’Farrell DA, Ng AK, Wang LC. Treatment of cutaneous metastases of Merkel cell carcinoma with surface-mold computer-optimized high-dose-rate brachytherapy. J Clin Oncol. accepted for publication.
ii) Use of PET-CTs in managing MCC
Dr. Wang shared some anecdotal cases from the Dana Farber on the use of PET/CTs for the management of MCC (220 PET/CTs in 91 patients). MCC is an FDG-avid tumor. PET/CTs appear to be useful in patients who present with nodal metastases with no identified primary. In those patients, upstaging as well identification of primary lesions on the skin have been observed. PET/CTs also appear to be useful in patients at high risk for recurrence/metastases. In those patients, identification of cutaneous metastases and deep lymph node metastases allowed for the earlier use of salvage therapy. Metabolic characterization of liver and bone metastases by PET/CT have been observed to precede image expression by CT.
There was no clear consensus among MMIG members as to the role of PET/CT in lower-risk patients (small primary lesions with negative nodal disease).
5) Development of an IL-12 intra-tumoral electroporation trial in MCC
Siegrid Yu, U of California, San Francisco
Dr. Yu briefly described the results of the pilot studies in which IL-12 plasmid DNA was injected into a cutaneous malignant melanoma lesion, followed by 1 second of electroporation using small needles connected to an electrical pulse generator. She spoke about the rationale for using IL-12 electroporation in cutaneous MCC tumors.
Results of pilot study in melanoma:
- Therapy was safe, well tolerated, and increased local IL-12 levels in the tumor
- No significant increase in systemic IL12 levels was observed
- 76% of lesions demonstrated necrosis within 31 days
- 4/19 patients with distant disease had distant response including 3 complete responses
Rationale for using IL12 electroporation in MCC:
- MCC is an immunogenic tumor
- MCC is likely associated with an immunosuppressive microenvironment
- Potential to increase immunogenicity of cancer cells
- Safe with good suggestion of efficacy in melanoma.
Sites of enrollment for the clinical trial (targeted for opening in 2010):
- Fred Hutchinson Cancer Research Center, Seattle
- University of California, San Francisco
In attendance at the Miami Beach 2010 MMIG meeting:
Maria S Alima (Colombia, South America)
Chris Bichakjian (U Michigan)
Jeremy Bordeaux (Case Western Reserve University) Issac Brownell (MSKCC, New York)
Jayasri Iyer (UW, Seattle)
Natalia Jaimes (Colombia, South America)
Shinichi Koba (UW, Seattle)
Miriam Lango (Fox Chase Cancer Research Ctr) Bob Lutz (ImmunoGen, Inc, Waltham MA)
Patrick Moore (U, Pittsburg)
Paul Nghiem (UW, Seattle)
James O’Leary (ImmunoGen, Inc)
Pablo Penas (U of Sydney, Westmead Hospital) Linda Wang (DF/BWCC, Boston, MA)
Richard Wang (UTSW, Dallas)
Kathy Whiteman (ImmunoGen, Inc, Waltham MA) Siegrid Yu (UCSF, San Francisco)
Nathalie Zeitouni (Roswell Park)
Frank Zhan (Beaver Medical Group, Highland CA)