Immunotherapy
Jump to Section:
Purpose of immunotherapy
Immunotherapy Immunotherapy A therapy that improves the function of the cells that recognize and destroy foreign objects in your body, such as a virus, bacteria or cancer. is a branch of medicine that uses therapies to augment the body’s own immune cells’ ability to recognize and destroy tumor cells. Immunotherapy is rapidly becoming a preferred systemic systemic Affecting the entire body. Compare to local. therapy in several cancer cancer A term used to describe diseases in which abnormal cells continually divide without normal regulation. Cancerous cells may invade surrounding tissues and may spread to other regions of the body via blood and the lymphatic system. types, especially because responses to immunotherapy (when they occur) are generally long-lasting. The durability of immunotherapy responses places this approach in stark contrast to chemotherapy chemotherapy Treatment with drugs to kill cancer cells or to render them less active. May be given intravenously or orally depending on the drug and situation. , which was previously considered the standard option for patients with metastatic metastatic Having to do with the spread of cancer from a primary site of origin to distant areas beyond the draining lymph nodes. MCC. One study of patients with metastatic MCC showed that while standard chemotherapy led to initial shrinkage of the tumors in the majority of patients, the shrinkage did not last long. By three months after starting chemotherapy, half of patients had recurrent growth of their tumors, and by ten months after starting chemotherapy, over 90% had tumor progression.1
Immune Checkpoint Inhibitors (ICI)
While there are several therapies that can stimulate the immune system, the most promising emerging option includes a class of drugs called the immune checkpoint inhibitors immune checkpoint inhibitors Immune checkpoint inhibitors (ICIs) are therapies that turns on the immune system by blocking an inhibitor (a 'checkpoint') that normally restrains the immune system. Such agents can sometimes cause the immune system to recognize and destroy a cancer. (ICIs). Tumors escape the immune system by pressing “brakes” (like the PD-1 protein) on the surface of the killer immune cells. The ICIs block these brakes from getting pressed and allow immune cells to function better. There are several ICIs that have been approved for treatment of advanced MCC. Side effects for such agents may include problems that arise if the immune system damages other parts of the body.
A recent study looked at a type of ICI called pembrolizumab (brand name Keytruda), which was administered as an intravenous infusion every three weeks in the outpatient clinic to patients with metastatic MCC, who had not received any prior systemic therapy. Of those patients, 56% had impressive shrinkage of their tumors. Patients with both virus-positive and virus-negative tumors responded to the treatment. Importantly, 86% of those who initially responded had long lasting responses, which were strikingly more durable than the chemotherapy responses (Nghiem et al, 2016). Based on these data, pembrolizumab was listed as a treatment option for patients with metastatic MCC in the 2017 NCCN NCCN National Comprehensive Cancer Network. The NCCN publishes annual consensus guidelines for the care of cancers, including MCC, based on expert opinion from all major cancer centers in the United States. guidelines. In 2018, pembrolizumab was approved by the FDA for treatment of MCC and can be prescribed by oncologists outside of clinical trials. In 2019, a follow-up study validated these early findings with encouraging long-term outcomes.
Avelumab (brand name Bavencio), another ICI that also works by blocking the PD-1 pathway (it binds to a ligand of PD-1 called PD-L1), is an FDA-approved drug that has shown considerable promise in fighting MCC. This drug was tested in 88 patients with metastatic MCC who had previously been treated with chemotherapy, and whose tumors had then come back. These patients thus had especially difficult-to-treat tumors. Of the 88 patients treated, 28 (32%) responded with significant tumor shrinkage. As with pembrolizumab, the responses appeared to be strikingly more durable than chemotherapy responses, with over 80% of patients who initially responded to avelumab having impressively durable responses continuing beyond a year.2 It has also been shown to be effective in patients with metastatic disease that has not been previously treated with chemotherapy.
Other ICIs, including nivolumab (brand name Opdivo) and ipilimumab (brand name YERVOY) are also in clinical trials in advanced MCC. In addition, there are several other immunotherapy approaches being investigated for MCC in clinical trials, including intra-tumoral injection approaches and infusion of immune cells (T-cells or Natural Killer cells). There is clearly a promising future for immunotherapies in the treatment of MCC.
Below is a list summarizing the ICIs used for MCC, their pharmaceutical manufacturer, and a description of the agent:
Ipilimumab (Yervoy)
- Pharmaceutical company: Bristol-Myers Squibb
- Description: Antibody Antibody A highly specific protein made by our bodies that can recognize and bind to an agent such as a bacteria or virus. against CTLA-4
Nivolumab (Opdivo)
- Pharmaceutical company: Bristol-Myers Squibb
- Description: Antibody against PD-1
Pembrolizumab (Keytruda)
- Pharmaceutical company: Merck
- Description: Antibody against PD-1
- FDA approved 12/19/2018
Avelumab (Bavencio)
- Pharmaceutical company: EMD Serono/Pfizer
- Description: Antibody against PD-L1
- FDA approved 3/23/2017
The following diagrams provide additional information about immunotherapy and ICIs used for MCC:
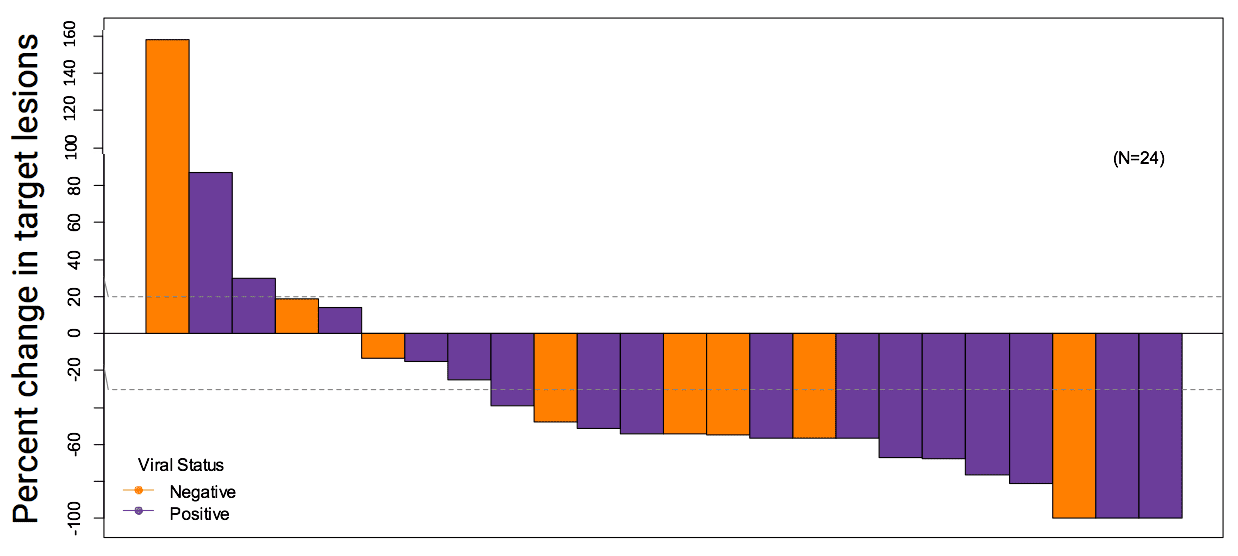
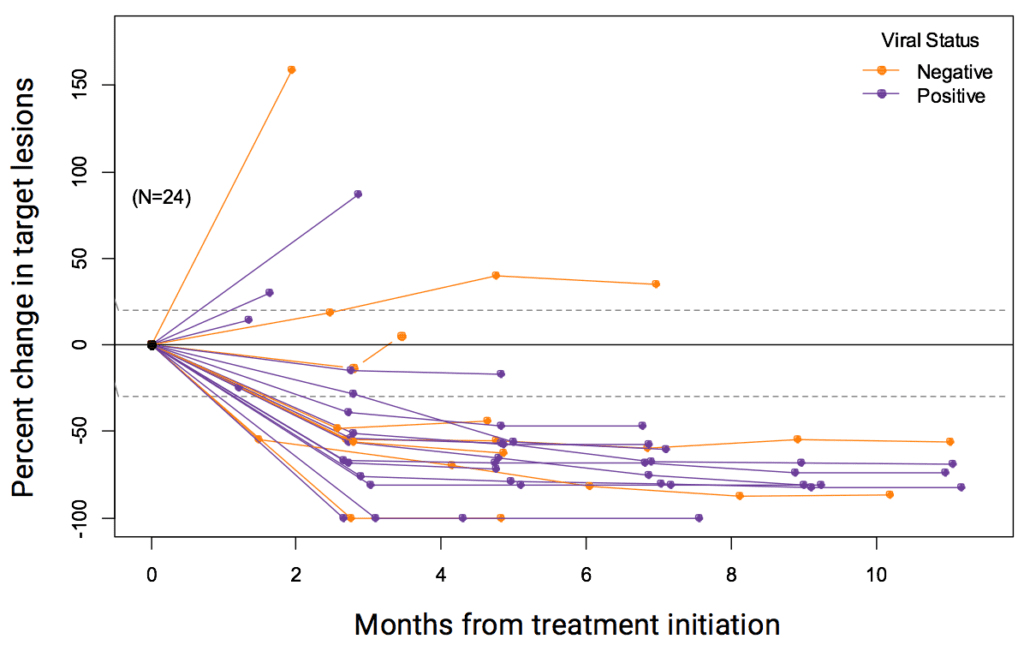
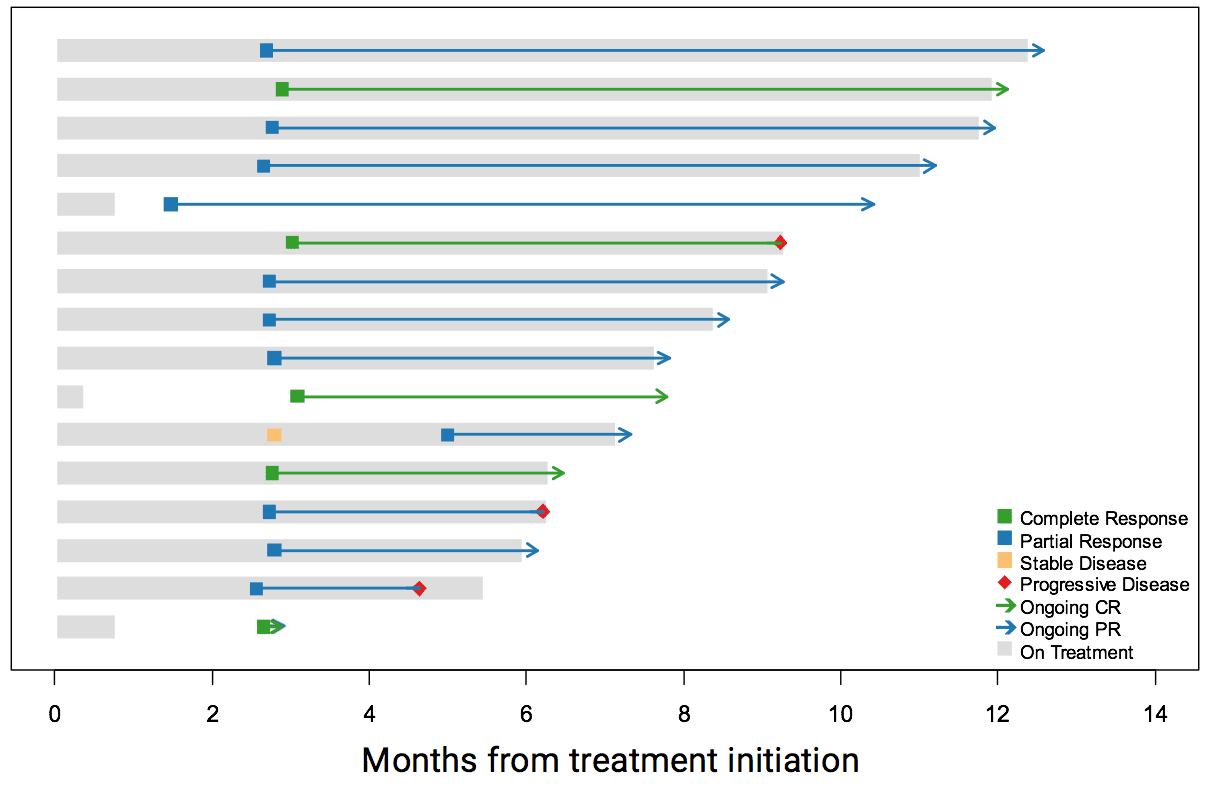
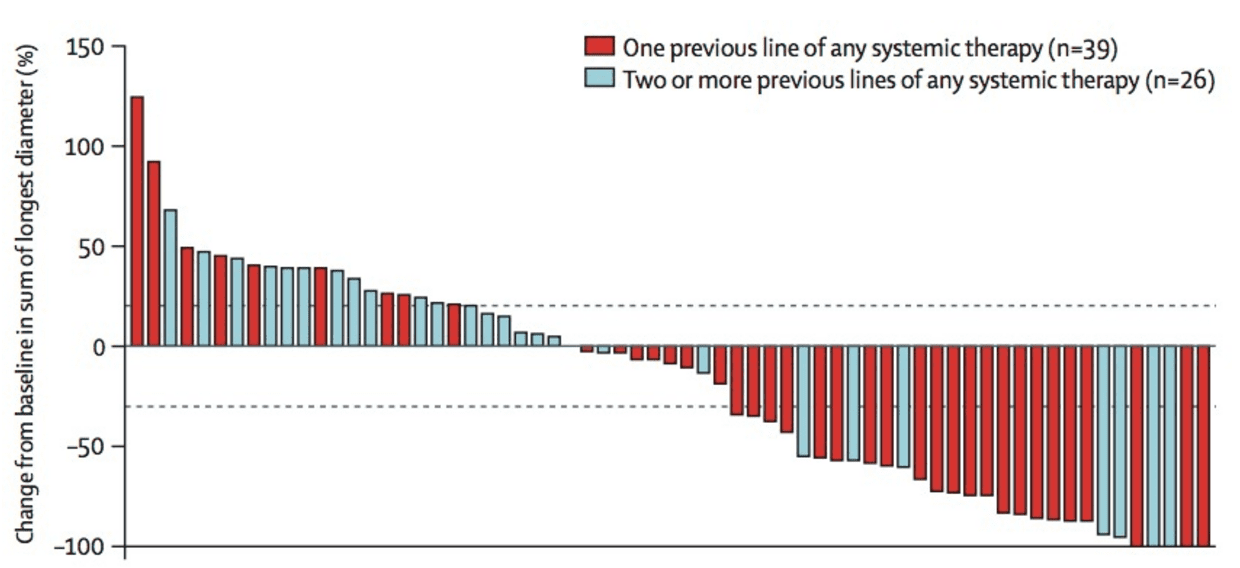
Who this works for
Patients with advanced MCC, such as those with distant metastases (stage IV) or MCC that cannot be removed surgically.
Who this doesn’t work for
Patients who have a suppressed immune system, which could be due to another cancer or uncontrolled HIV, or prior organ transplant or serious autoimmune disease that requires immunosuppressive drugs.
Side effects
Common side effects for immune checkpoint inhibitors include, but are not limited to, fatigue, cough, nausea, and itching. The vast majority of patients report good quality-of-life while receiving these drugs, which typically do not cause hair loss, nausea, vomiting, infections, etc., in striking contrast to the older chemotherapy options. However, ICIs can sometimes cause severe, life-threatening side-effects, related to immune attack against normal body organs. These include colitis (diarrhea), hepatitis (liver injury), pneumonitis (lung inflammation), hormone changes, nerve damage, etc. Close follow-up and contact with the treating oncologist is critical to avoid serious injury.
Often used in conjunction with
Radiation, T-cells
What to do next
There is currently a multicenter clinical trial recruiting patients who have had MCC and are at risk for disease recurrence, for more information click here
Please visit the Clinical trials page and the National Institutes of Health’s website to learn more about active MCC clinical trials, their eligibility, and location sites.
FAQs
How could a patient get access to an Immune checkpoint inhibitor for MCC?
Immune checkpoint inhibitors are given through clinical trials, off-label, or compassionate use (where the pharmaceutical companies pay for the therapy).
What immune checkpoint inhibitors are FDA-approved for MCC?
Avelumab (Bavencio) is the first and only FDA-approved immunotherapy drug for MCC. Avelumab has received orphan drug and breakthrough drug status for its use in MCC. Pembrolizumab was recently listed as a treatment option for patients with metastatic MCC on the NCCN guidelines. This drug is approved by the US FDA for treatment of other cancers and can be prescribed by oncologists outside of clinical trials.
Footnotes
- 1Iyer JG, Blom A, Doumani R, et al. Response rates and durability of chemotherapy among 62 patients with metastatic Merkel cell carcinoma. Cancer Med. 2016;5(9):2294-2301. doi:10.1002/cam4.815
- 2Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17(10):1374-1385. doi:10.1016/S1470-2045(16)30364-3
- 3Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med. 2016;374(26):2542-2552. doi:10.1056/NEJMoa1603702
Clinical Publications
The following clinical publications and scientific research provide additional in-depth information about immunotherapy.