Adjuvant Avelumab in Merkel Cell Carcinoma Trial
• What is the purpose of the ADAM trial?
The ADAM (Adjuvant Avelumab in Merkel) trial aims to study the effectiveness of
immunotherapy
immunotherapy
A therapy that improves the function of the cells that recognize and destroy foreign objects in your body, such as a virus, bacteria or cancer.
with Avelumab towards reducing the risk of
Merkel cell
Merkel cell
Merkel cells are found in the lower part of the epidermis. Although the exact function of Merkel cells is unknown, they are thought to be touch receptors. Also known as neuroendocrine cells, they have machinery similar to nerve cells and to hormone-secreting (endocrine) cells.
cancer
cancer
A term used to describe diseases in which abnormal cells continually divide without normal regulation. Cancerous cells may invade surrounding tissues and may spread to other regions of the body via blood and the lymphatic system.
coming back. This trial is designed for subjects who have
Merkel Cell Carcinoma
Merkel Cell Carcinoma
A skin cancer composed of cells that look microscopically similar to normal Merkel cells present in the skin. MCC was first described in 1972 and only in the 1990s was the CK20 antibody developed to make it easily identifiable by pathologists. Many doctors and patients are not aware of this cancer because of its recent description and relative rarity (~2,000 cases/year in the US--roughly 30 times less common than melanoma). About 40% of patients treated for MCC will experience a recurrence, making it far more aggressive than most other types of skin cancer, including melanoma.
(MCC) that has travelled to the lymph nodes; these patients are at very high risk of the cancer coming back. The study team would give the study drug to subjects after they have had surgery, radiation, or both treatments, and see if it might prevent the cancer from coming back. Currently, the standard approach for these high-risk patients is just surveillance without any active drug treatment.
• If Avelumab is already approved, why is this Phase 3 Clinical Trial needed?
Avelumab is currently approved by the Food and Drug Administration (FDA) in the United States for the treatment of metastatic metastatic Having to do with the spread of cancer from a primary site of origin to distant areas beyond the draining lymph nodes. MCC that has travelled to distant organs. However, it has not yet been proven to reduce the risk of cancer coming back in patients who have MCC in the lymph nodes only. Hence, in the ADAM Trial, Avelumab is an experimental drug as it has not been approved for treatment of MCC that has travelled only to the regional lymph nodes.
• How do I know if I am eligible?
Eligible patients have MCC that has travelled to the regional lymph nodes, and have been treated with surgery, radiation therapy radiation therapy The use of radiation to kill cancer cells and shrink tumors. Merkel cell carcinoma is a highly radiation sensitive cancer in most cases. or both treatments. Your study doctor will provide you more detailed information about your final eligibility.
• Where is the closest treatment center for me?
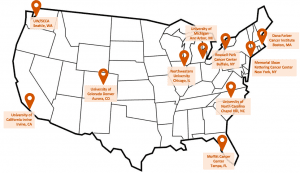
There are 10 research cancer centers across the United States participating in the ADAM Trial.
- University of Washington, Seattle WA
- University of Michigan, Ann Arbor MI
- Dana Farber Cancer Institute, Boston, MA
- Roswell Park Comprehensive Cancer Center, Buffalo NY
- Moffitt Comprehensive Cancer Center, Tampa FL
- Northwestern University/Robert H. Lurie Comprehensive Cancer Center, Chicago IL
- Memorial Sloan Kettering Comprehensive Cancer Center, New York NY
- University of Colorado Cancer Center, Aurora CO
- University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill NC
- University of California Irvine Chao Family Comprehensive Cancer Center, Orange CA
• How will getting placebo affect my health?
In the ADAM trial, half the patients get avelumab and the other half get placebo (normal saline solution). This allows the researchers to compare the benefits and risks of avelumab against the current standard treatment option of surveillance. Both groups in the trial get a similar intensive schedule of surveillance that can help detect the cancer coming back. Hence, placebo group could also benefit from participating in this trial. Infusion of the saline solution itself has minimal risks.
• What are the potential risks of participating in this trial?
You might have side effects related to the study treatment (avelumab). Since avelumab is FDA-approved and has already been given to thousands of patients, we are quite familiar with its side-effects. Reactions, including allergic reactions, can happen during or following the infusion and are mostly mild or moderate. These include chills, fever, muscle pain, shortness of breath and a decrease or increase in blood pressure. Since avelumab works by stimulating the immune system, it can cause autoimmune side-effects, such as inflammation in organs like colon, liver, lung and hormone-producing galnds, In some cases, these side-effects may be severe that could require intensive medical support, or it could lead to death. As well, we may not know all the risks of this treatment. However, your study team will monitor your wellbeing closely during your participation in the trial and ensure that you receive the appropriate care. Your study doctor will also discuss with you in details about the risks associated with Avelumab.
• Are there costs associated with this trial?
If you decide to join this study, the study treatment (Avelumab or placebo) will be provided to you at no cost. But you may incur costs associated with your medical care, such as doctor’s visits, laboratory tests, scans and any other medical care needed, including treatment of side effects. You or your insurance company will be responsible for these costs. Your study team can help find out whether your insurance company will cover these costs.
• Can I stop the clinical trial at any time?
Participating in the ADAM Trial is voluntary. You can change your mind and stop at any time. You will need to inform the study team so that you end the study in the safest way.
• What will happen if I stop the trial?
You do not lose any benefits you receive now or have a right to receive. The study team will discuss different withdrawal or alternative options and your responsibilities with you. They may also talk to you about follow-up care, if needed. Information collected during your participation in the clinical trial will still be apart of the research. However, information that identifies you will not be used. You can ask the study team how they plan to use your information and how they will protect it.
• What will happen when the study ends?
The study team will find out how well subjects have responded to Avelumab. Information collected from you during your participation in the trial will be kept by the study team institution and may be used for future MCC research. However, information that identifies you will not be shared. You can ask the study team how they plan to use your information and how they will protect it.
• Who can I contact to find out more information on this trial?
You could choose to contact a study site closest to your location. You can also call the central sponsor team of ADAM trial by email ([email protected]) or phone (206-606-1795). Your
oncologist
oncologist
A doctor who specializes in treating cancer. Three main types of oncologists exist: radiation oncologist, medical oncologist, and surgical oncologist.
should also be able to help connect you with the study team members. If you would like to share information about the trial with your friends and family, please see link to our study flyer.