Avelumab in patients with previously treated metastatic Merkel cell carcinoma (JAVELIN Merkel 200): updated overall survival data after >5 years of follow-up
January 24, 2022
Merkelcell.org Summary
This study presents updated findings from the JAVELIN Merkel 200 trial after more than 5 years of follow-up. It clearly shows that patients with metastatic MCC (mMCC) who previously experienced disease progression following chemotherapy had meaningful long-term overall survival, unlike for chemotherapy. These data further support the role of avelumab as a standard of care for patients with mMCC.
Abstract
Background
Merkel cell carcinoma (MCC) is a rare, aggressive skin cancer that has a poor prognosis in patients with advanced disease. Avelumab [anti-programmed death-ligand 1 (PD-L1)] became the first approved treatment for patients with metastatic MCC (mMCC), based on efficacy and safety data observed in the JAVELIN Merkel 200 trial. We report long-term overall survival (OS) data after >5 years of follow-up from the cohort of patients with mMCC whose disease had progressed after one or more prior lines of chemotherapy.
Patients and methods
In Part A of the single-arm, open-label, phase II JAVELIN Merkel 200 trial, patients with mMCC that had progressed following one or more prior lines of chemotherapy received avelumab 10 mg/kg by intravenous infusion every 2 weeks until confirmed disease progression, unacceptable toxicity, or withdrawal. In this analysis, long-term OS was analyzed.
Results
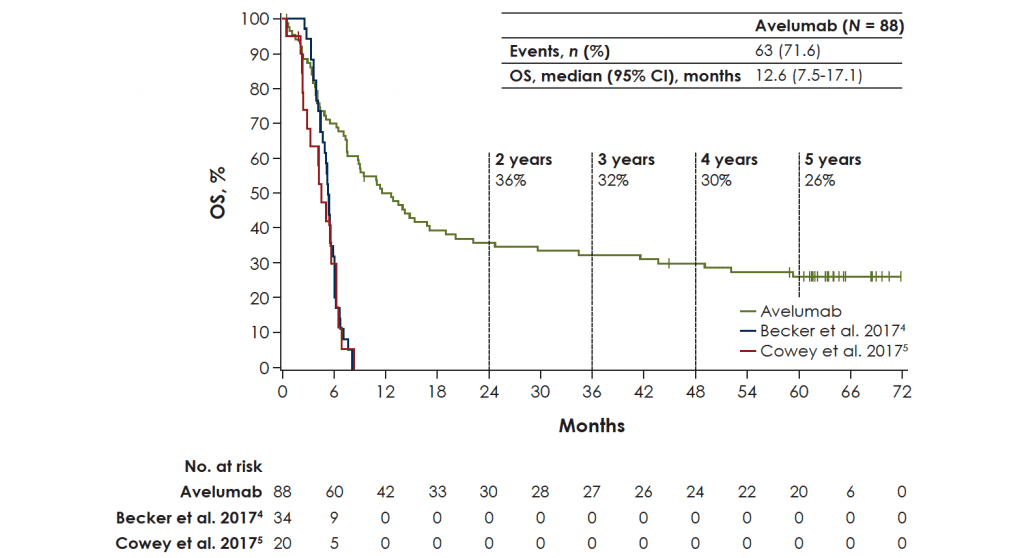
In total, 88 patients were treated with avelumab. At data cut-off (25 September 2020), median follow-up was 65.1 months (range 60.8-74.1 months). One patient (1.1%) remained on treatment, and an additional patient (1.1%) had reinitiated avelumab after previously discontinuing treatment. Median OS was 12.6 months [95% confidence interval (CI) 7.5-17.1 months], with a 5-year OS rate of 26% (95% CI 17% to 36%). In patients with PD-L1+ versus PD-L1− tumors, median OS was 12.9 months (95% CI 8.7-29.6 months) versus 7.3 months (95% CI 3.4-14.0 months), and the 5-year OS rate was 28% (95% CI 17% to 40%) versus 19% (95% CI 5% to 40%), respectively (HR 0.67; 95% CI 0.36-1.25).
Conclusion
Avelumab monotherapy resulted in meaningful long-term OS in patients with mMCC whose disease had progressed following chemotherapy. These results further support the role of avelumab as a standard of care for patients with mMCC.